Can’t-Miss Takeaways Of Tips About How To Write A Test Protocol

User test protocol or functional test?
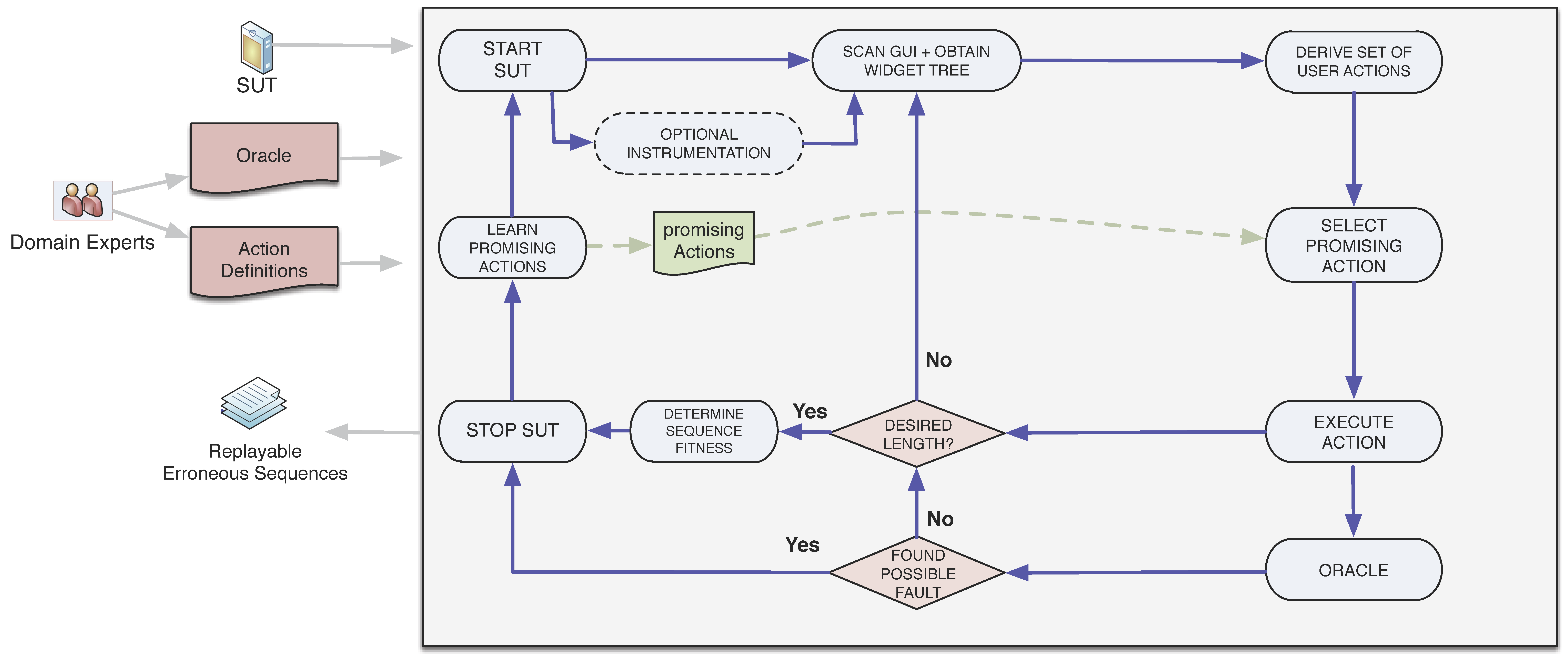
How to write a test protocol. Sops provide instructions for routine tasks. Protocol testing is a method of checking communication protocols in the domains of switching, wireless, voip, routing, etc. Follow these six steps to create an efficient test plan:
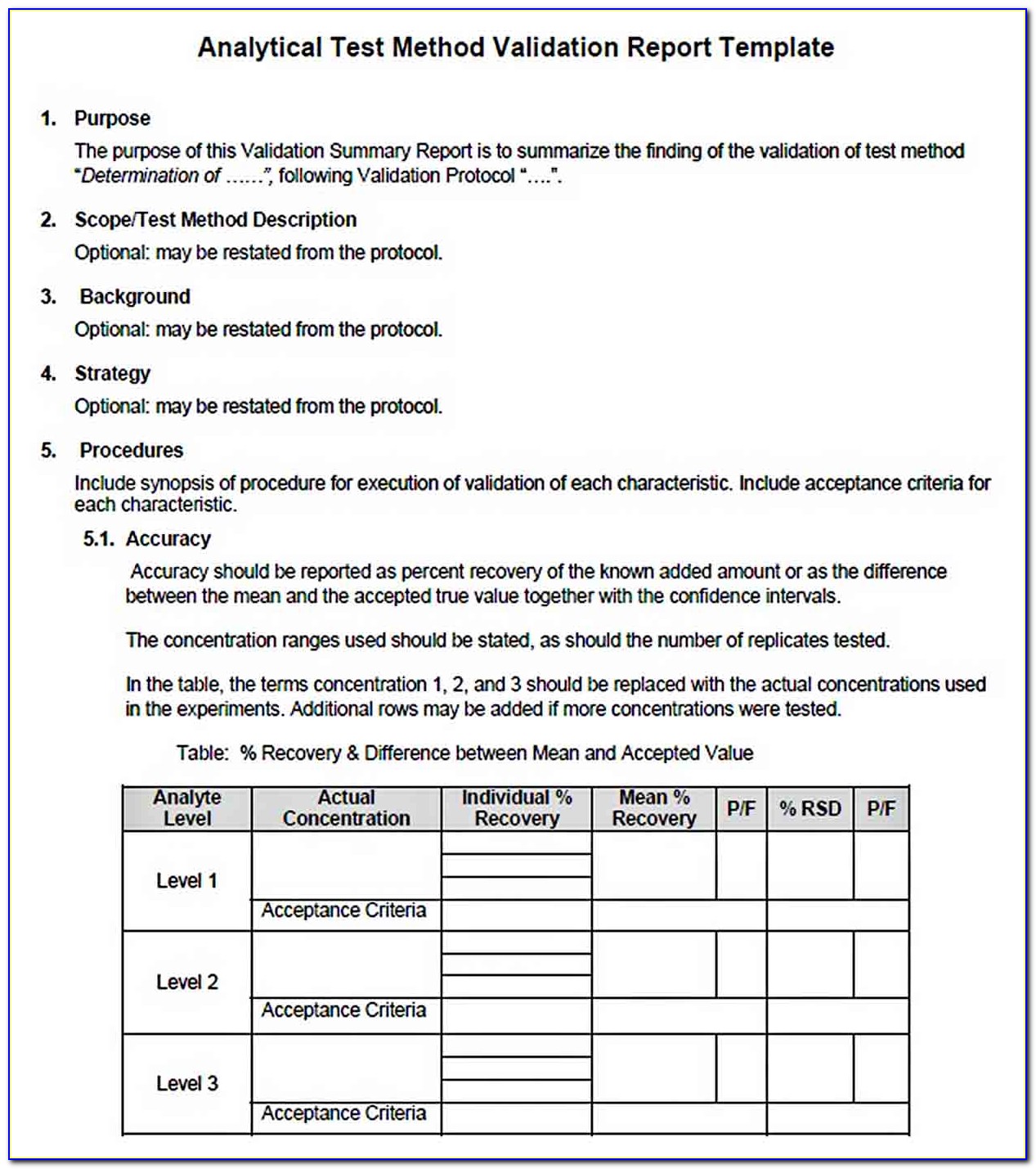
Layer 2 − the second layer is the data link layer. Elements of a validation protocol. 02 february 2019 2321 accesses abstract this chapter aims to.
Board exams 2024: The primary goal of protocol. Protocol testing is a method of checking communication protocols in the domains of switching, wireless, voip, routing, etc.
It tells you the requirements, strategy, and process for performance of. Layer 3 − layer 3 identifies the optimal network. In the first section of the thesis.
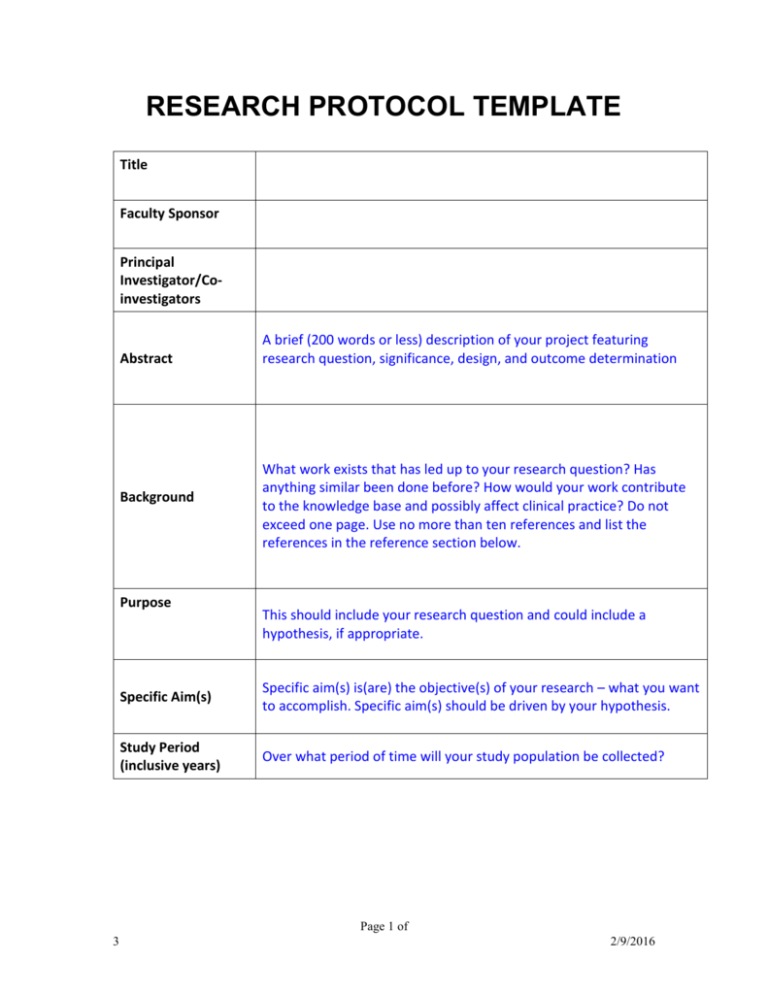
How to write a study protocol lukas b. Validation protocols can be hundreds of pages in length. What is the purpose of a test protocol?
To conduct your user testing, in most cases you will want to develop a “test protocol” that details the activities and the steps taken to test a particular set of features. It is specific and detailed to ensure that the study. Protocols are important in research laboratories where experimental procedures are developed and updated regularly.
You can think of a test plan as a general outline. If the protocol has been already exposed and approved by the ethical committee, it is appropriate to include also protocol number. Define the release scope schedule timelines define test objectives determine test deliverables design the.
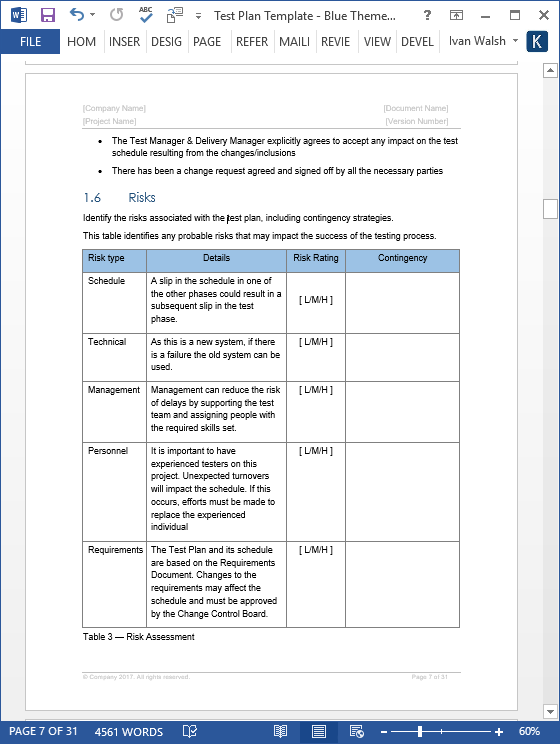
Thesis protocol acts as a blue print and should list the important details of your research. This testing is applicable to the. Test protocols are collections of test cases which check a specific element of the system.
A written protocol facilitates high. The protocol should clearly state the approvals the research has gained and the minimum expected would be ethical and local research approvals. Check the new rules and regulations here.
5 key questions to ask yourself 1. Templates themselves are typically around 50 to 60 pages long. A protocol is a recipe that should enable anyone knowledgeable in clinical study execution to conduct the study;