Neat Tips About How To Tell If Its Ionic Or Covalent

Identify the following compounds as wither ionic or covalent:
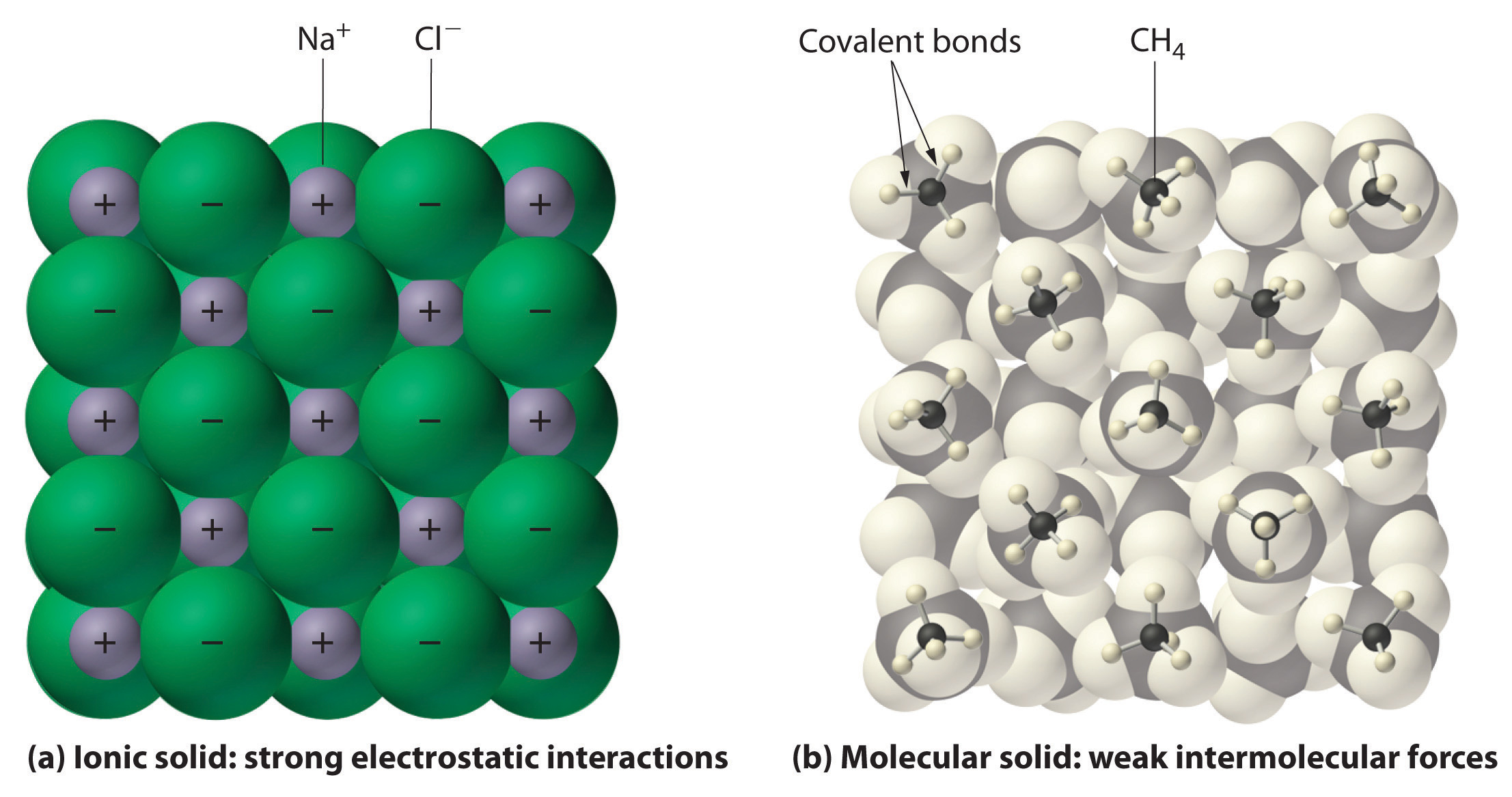
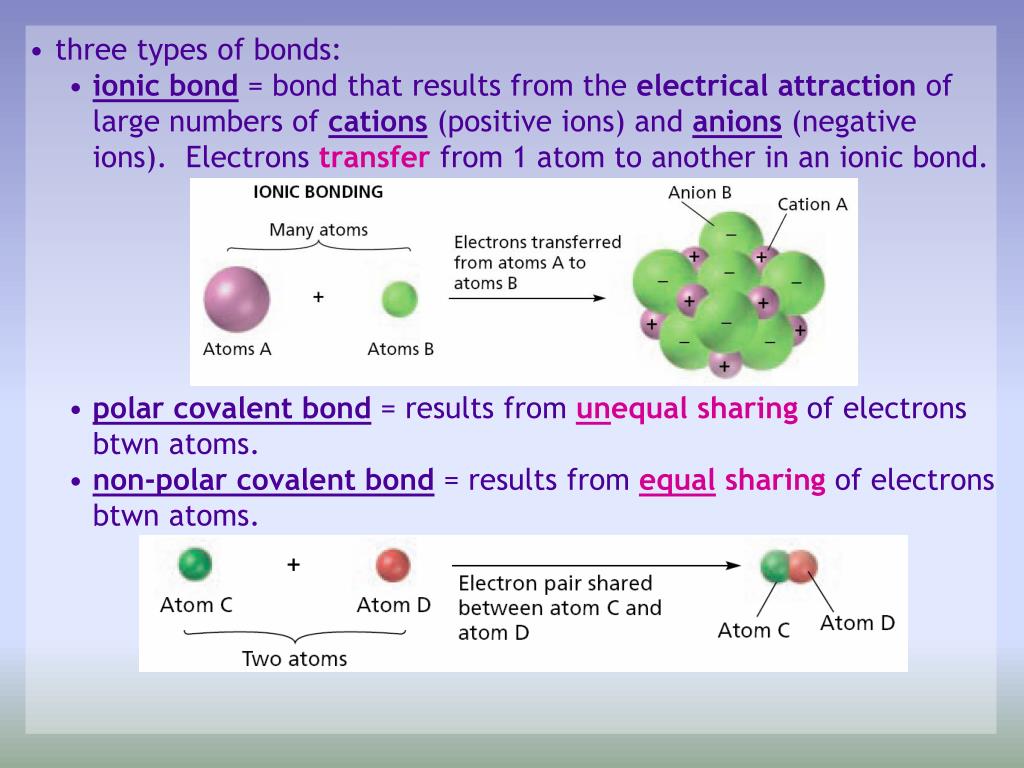
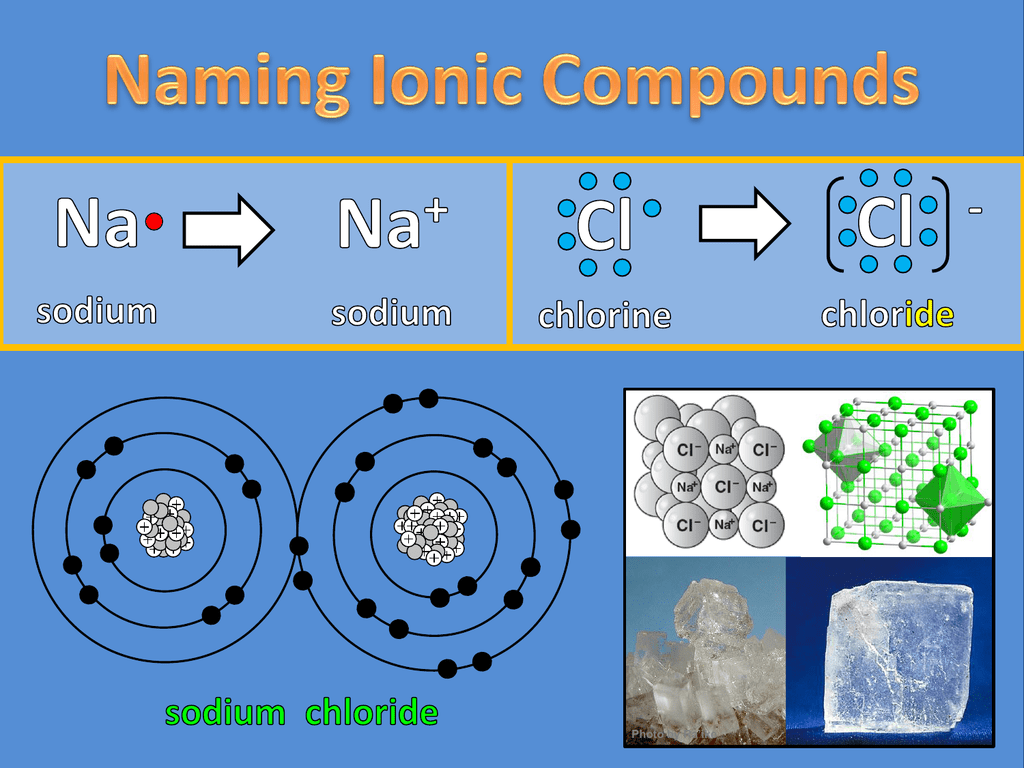
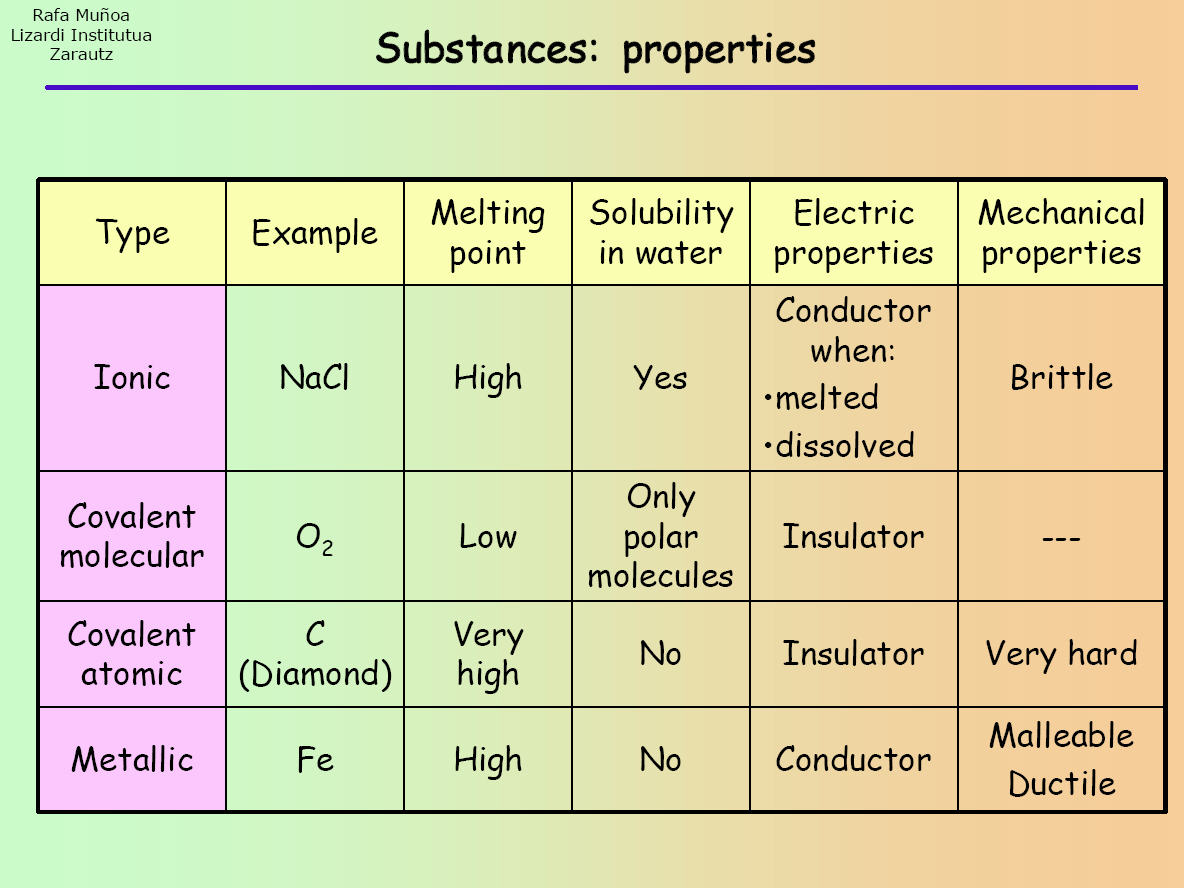
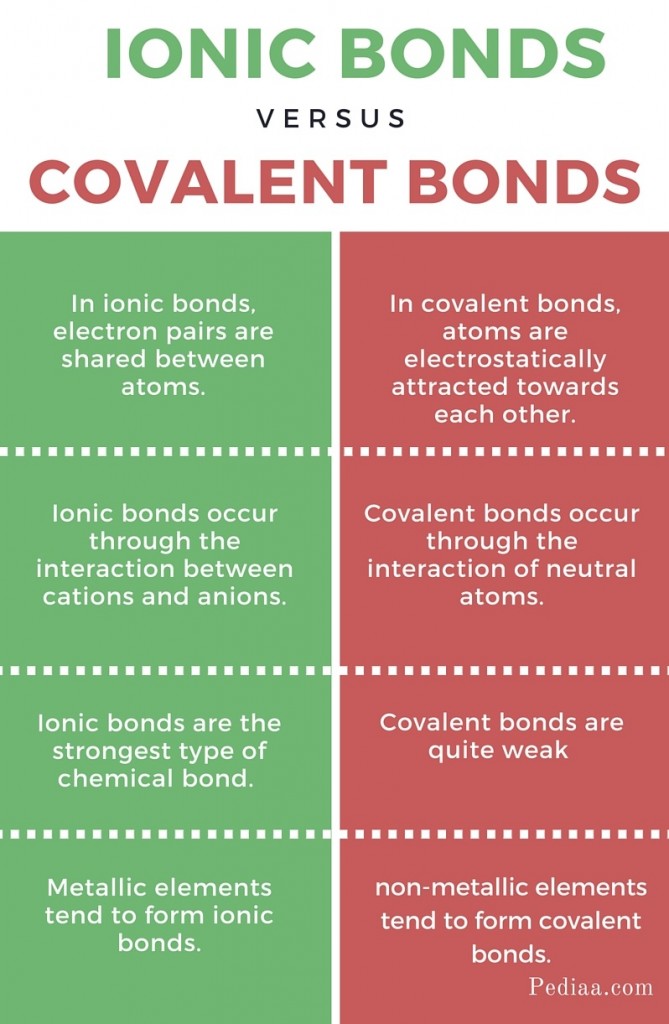
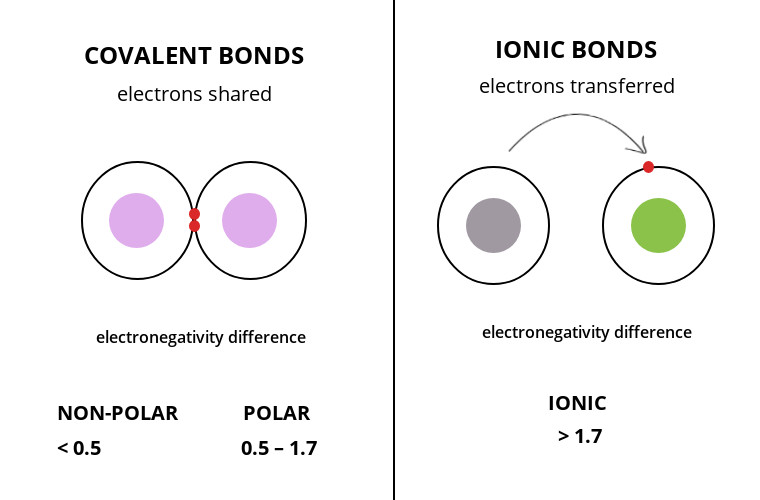
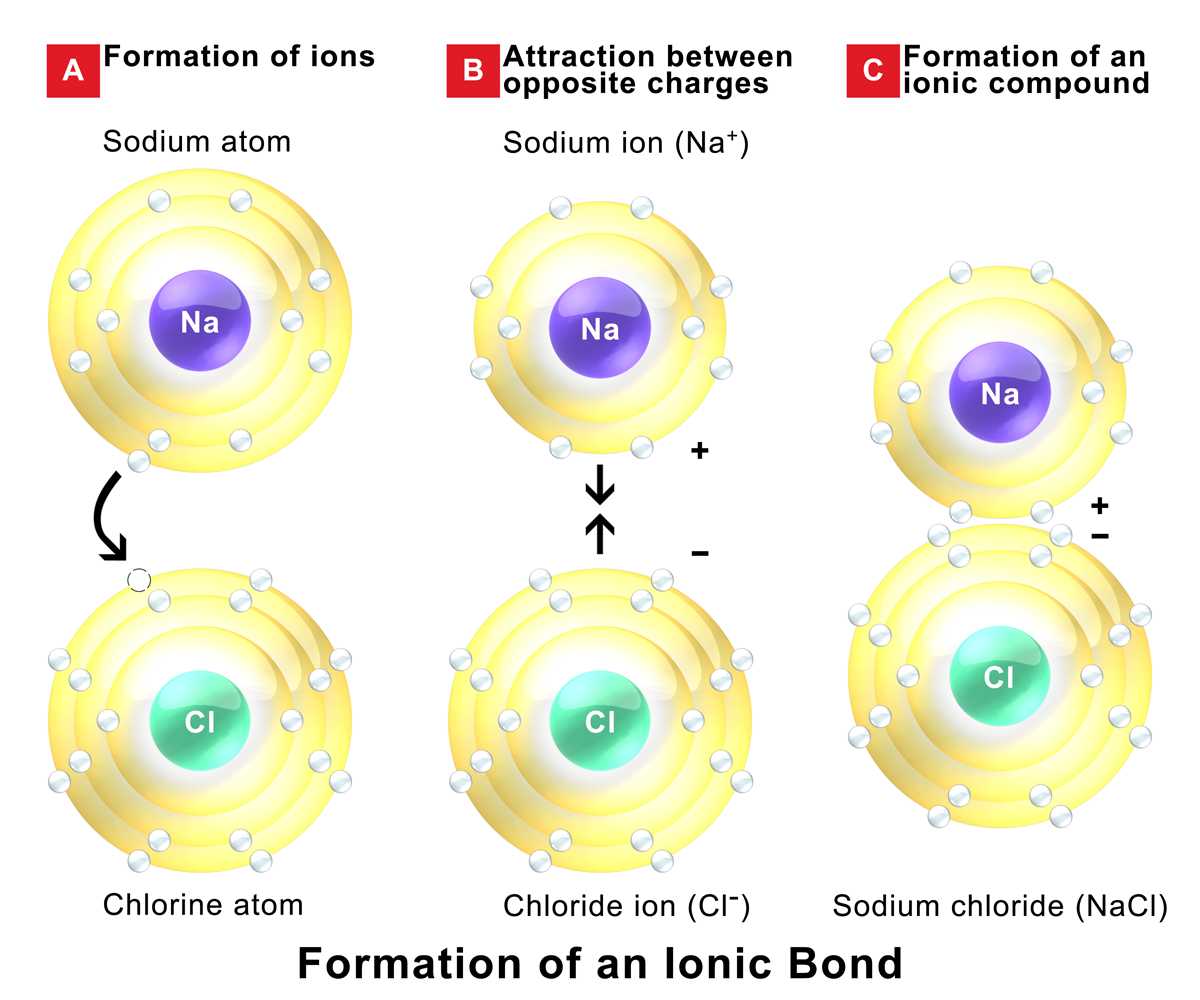
How to tell if its ionic or covalent. In ionic bonding, atoms transfer electrons to each other. A bond in which the electronegativity difference between the atoms is between 0.5 and 2.1 is called a polar covalent bond. In an ionic bond, an electron is given from one atom to another.
How to determine whether a compound is ionic, covalent, or an acid. H 2 o 2, the bleach and disinfectant hydrogen peroxide. Ionic bonds require at least one electron donor and one electron acceptor.
First, a sample of the unknown substance can be placed in a test tube and put over a flame. When the difference is very small or zero, the bond is covalent and nonpolar. This is done to determine the melting point.
The difference with a polar covalent. The formation of ionic bond takes place when. You have to calculate the difference in.
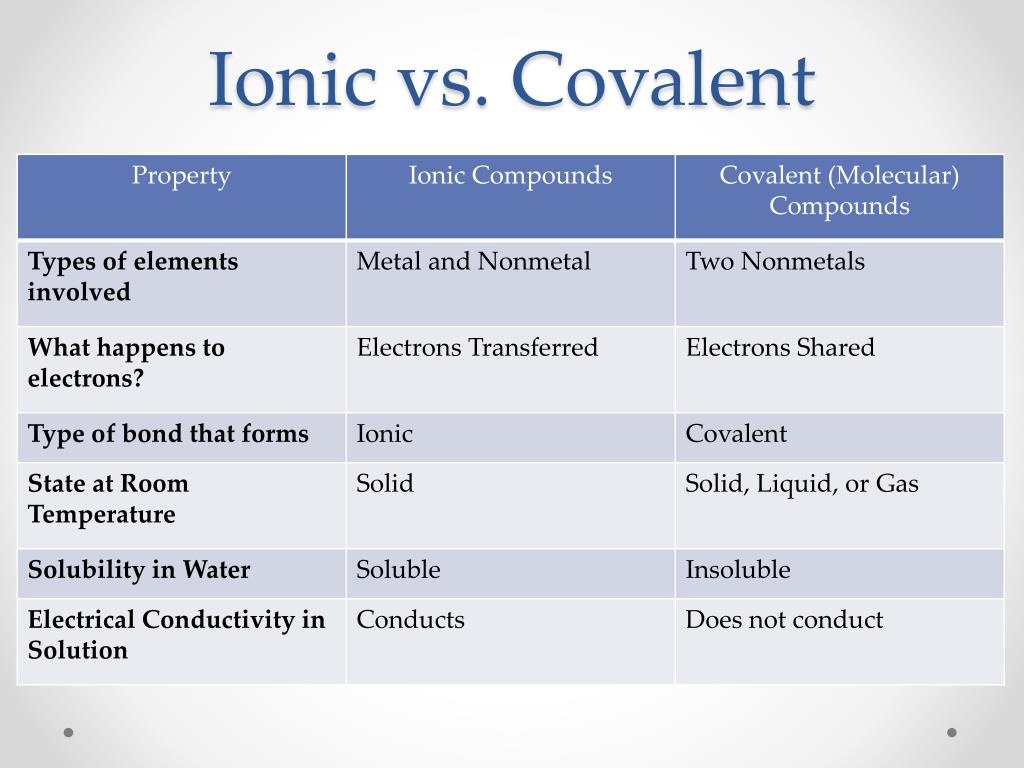
A polar covalent bond is a. Ionic compounds can be of the following types: Compounds that do not contain ions, but instead consist of atoms bonded tightly together in molecules.
Another way to create bond is if those elements steal electrons from each other. For naming these compounds, watch the naming videos!ionic:. How to tell if a bond is ionic, covalent or polar covalent.
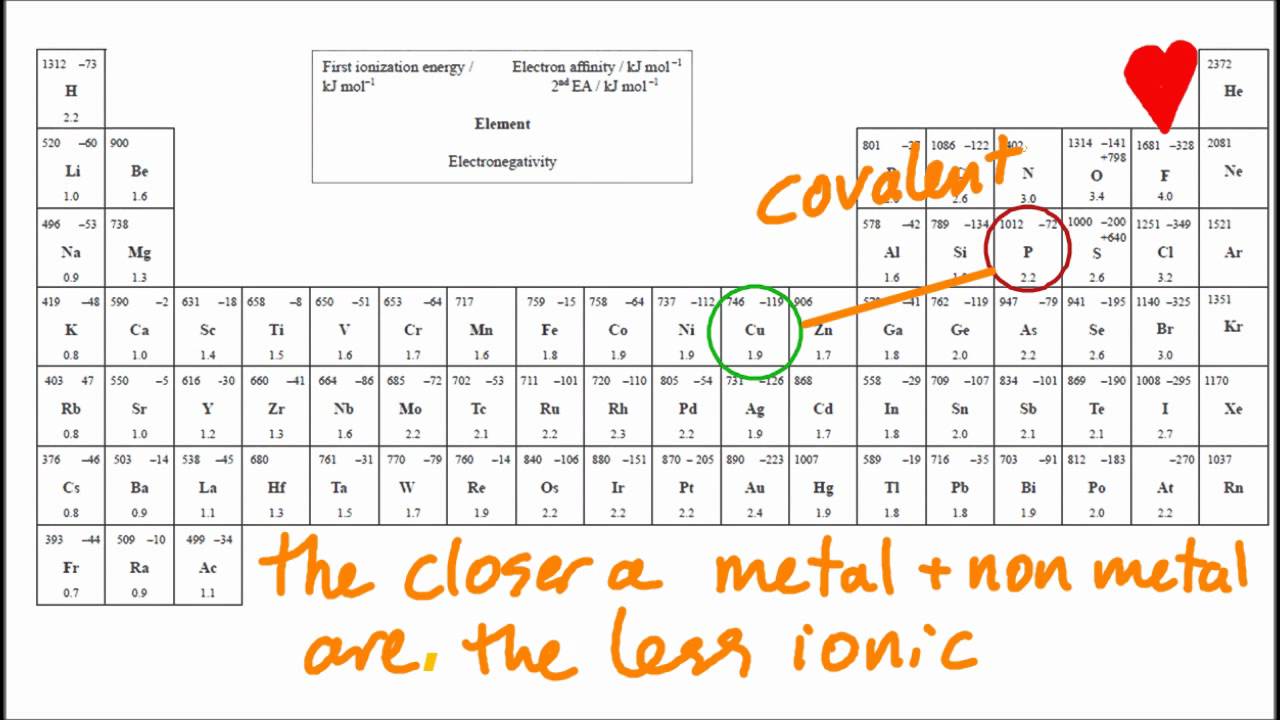
The absolute values of the electronegativity. Ionic compounds generally form from metals and nonmetals. Covalent bonds, on the other hand,.
The ionic bonds form between at least one metal and at least one non. In this section, you will learn about the bond strength of covalent bonds, and then compare that to the strength of ionic bonds, which is related to the lattice energy of a compound. If the difference of the electronegativity between the two elements is greater than 1.7 then the bond is ionic.
364k views 11 years ago. Ki, the compound used as a source of iodine in table salt. To figure out whether an element is a metal or a nonmetal,.
When it is large, the bond is polar covalent or ionic. How to identify an ionic vs covalent compound? In contrast, atoms with the same.